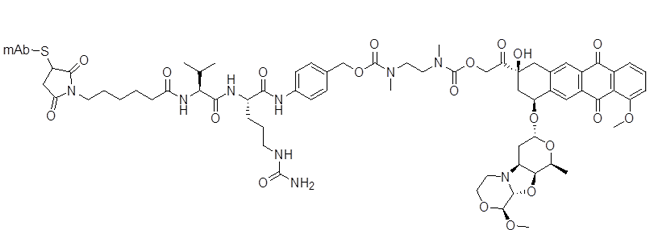Request a Quote

Linkers for ADCs

Linkers for Bioconjugation: Linkers are an important part of a successful antibody-drug conjugate (ADC). Most ADCs are referred to with the payload and linker as one unit such as MC-MMAF, MC-Val-Cit-Pabc-MMAE, SPDB-DM4, and SMCC-DM1. Linker-payloads reflect how each evolved with the knowledge at the time. Linker technologies were associated with a payload class and were usually combined and used as such. However, both constructs are independent and there is no reason why, for instance, an auristatin cannot be coupled with a pH-sensitive linker or a disulfide linker. This section will describe options for linkers and how NJ Bio can help with selection of a linker for a selected payload.
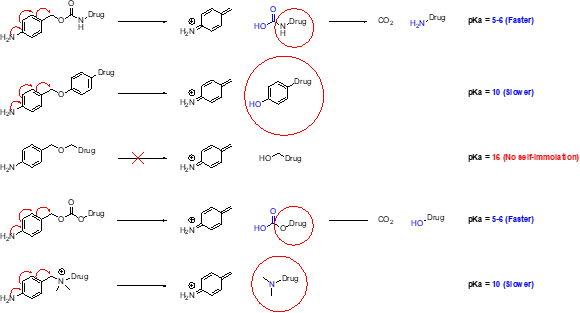
Types of Linkers: Linkers are divided into cleavable and non-cleavable types. Cleavable linkers have a biological weak point that releases a metabolite either by reduction, hydrolysis, or proteolysis. Common cleavable linkers are MC-Val-Cit-Pabc, disulfides (e.g., SPDB, SPP), and hydrazone for pH-sensitivity. Many cleavable linkers use a self-immolating mechanism to release the intact payload. It is important to note that the pKa of the leaving group will be important to drive the self-immolation.
Disulfide linkers are also cleavable in the sense that they break in the presence of high levels of free cysteine or glutathione found in the cytosol of cells. Disulfides are interesting constructs, as the kinetics of release can be modulated by the steric hindrance around the disulfides. Another advantage of disulfides is that they can be attached directly to the cysteine of the antibody, resulting in a cleavable linker with fewer atoms added to the drug-linker complex. An example of this is SPDB-DM4. Newer disulfides have also been made to release alcohols.
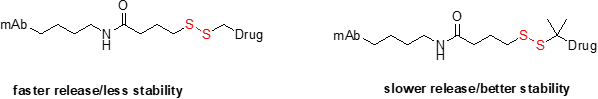
Finally, examples of pH-sensitive linkers are usually found in hydrazone-based linkers that can hydrolyze in the more acidic environment of the lysosome. Carbonates have also been found to be pH-sensitive, as seen in the CL2A linker of SN38. The same release kinetics and metabolite in cells were shown with and without the cleavable sequence. The disadvantage of carbonates and hydrazones is that they do deconjugate in circulation. These linkers have received less attention because deconjugation can lead to toxicity. However, three currently approved ADCs have pH-sensitive linkers.
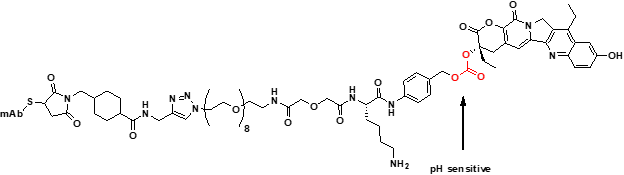
Cl2A Linker

Hydrazone
Non-cleavable linkers do not have a weak point and release from the antibody after complete lysosomal degradation of the ADC. Non-cleavable linkers are known for their stability and only release when the ADC completely degrades. The linker is part of the active metabolite as it stays attached to the payload and thus can change the property of the payload. A payload that has bystander activity can lose its bystander activity with a non-cleavable linker, which can also change the affinity of the payload to PGP pumps. Overall, non-cleavable linkers can change the activity of the active metabolite, which can impact activity, toxicity, and trafficking.
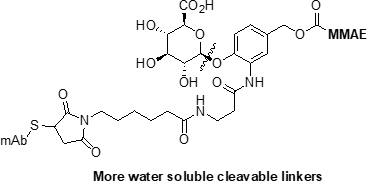
Finally, all types of linkers have had modifications to modulate the hydrophobicity, DAR and conjugation technology to create novel ADC constructs. Popular modified linkers are ones incorporating sugars, PEGS and enzymatic/click type conjugation. This additional solubility and DAR control can improve the therapeutic index by resulting in a better PK profile.
NJ Bio can design and select the right linker for your ADCs. Linkers can be mixed and matched with payload technology and optimized for your particular target, which can be a powerful way to enhance the overall success of your program. Contact NJ Bio to see how we can help with your linker needs.
The table below shows combinations of drug-linkers that have been used. Most of these can be adapted to different drug combinations, but these examples provide a visual representation of the structures of the most advanced and interesting drug-linkers.
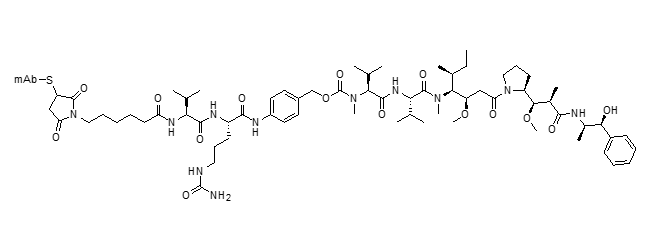
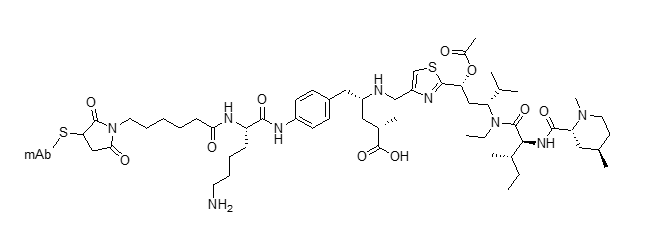
mc-vc-PABC-MMAE ADC (vedotin)
Clin. Cancer Res. 2012, 18, 5845–5849
https://DOI: 10.1158/1078-0432.CCR-12-1803
Blood 2018, 132 (Suppl. 1), 1683 DOI: 10.1182/blood-2018-99-118551
Cancer Res. 2016, 76, 3003–3013 DOI: 10.1158/0008-5472.can-15-1313
J. Clin. Oncol. 2018, 36, 3298–3306 DOI: 10.1200/JCO.2018.78.7697
Cancer Res. 2014, 74, 1214–1226 DOI: 10.1158/0008-5472.CAN-13-2440
Eur. J. Pharm. Sci. 2016, 93, 274–286 DOI: 10.1016/j.ejps.2016.08.015
Mol. Cancer Ther. 2014, 13, 2991–3000 DOI: 10.1158/1535-7163.MCT-13-0896
Nat. Med. 2018, 24, 203–212 DOI: 10.1038/nm.4472
Cancer Res. 2018, 78, 4059–4072 DOI: 10.1158/0008-5472.CAN-18-0327
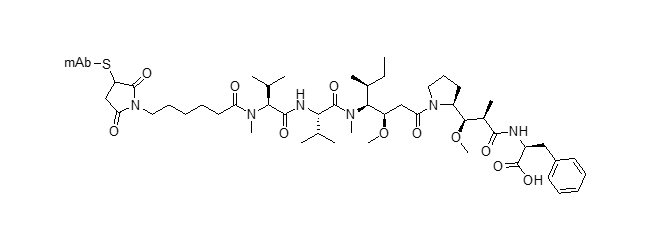
SMCC-DM1 ADC (emtansine)
J. Med. Chem. 2014, 57, 6949–6964. DOI: 10.1021/jm500766w
Future Oncol. 2013, 9, 319–326 DOI: 10.2217/fon.13.7
Adv. Med. Oncol. 2014, 6, 202–209 DOI: 10.1177/1758834014539183
N. Engl. J. Med. 2019, 380, 617–628 DOI: 10.1056/NEJMoa1814017
J. Exp. Clin. Cancer Res. 2016, 35, 104 DOI: 10.1186/s13046-016-0380-5
BMC Cancer 2019, 19, 517 DOI: 10.1186/s12885-019-5687-0
Invest. New Drugs 2018, 36, 869– 876 DOI: 10.1007/s10637-018-0570-4
mAbs 2019, 11, 1149–1161 DOI: 10.1080/19420862.2019.1618674
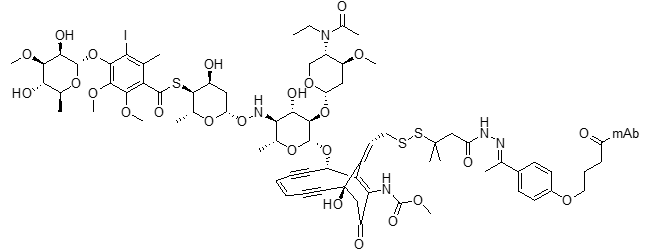
N-Acetyl-g-calicheamicin 1,2-dimethylhydrazine ADC (ozogamycin)
Clin Cancer Res. 2001, 7, 1490–1496
Clin Cancer Res 2018, 24, 3242–3246 DOI: 10.1158/1078-0432.CCR-17-3179
Oncologist 2018, 23, 1103–1108 DOI: 10.1634/theoncologist.2017-0604
Blood 2010, 116, 2618–2619 DOI: 10.1182/blood-2010-08-300871
Blood 2017, 130, 2373–2376 DOI: 10.1182/blood-2017-09-797712
Drugs 2017, 77, 1603–1610 DOI: 10.1007/s40265-017-0802-5
mc-MMAF ADC (mafodotin)
Clin. Cancer Res. 2018, 24, 4399–4406 DOI: 10.1158/1078-0432.CCR-18-0481
Blood Cancer J. 2019, 9, 37 DOI: 10.1038/s41408-019-0196-6
Blood 2014, 123, 3128–3138 DOI: 10.1182/blood-2013-10-535088
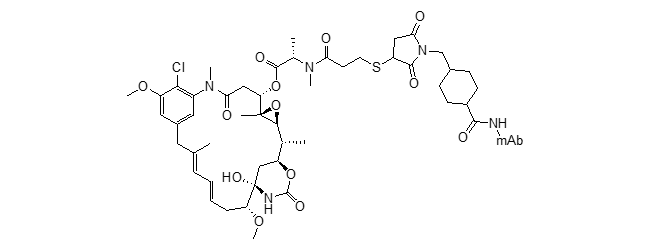
sulfo-SPDB-DM4 ADC (soravtansine)
Mol. Cancer Ther. 2015, 14, 1605–1613 DOI: 10.1158/1535-7163.MCT-14-1095
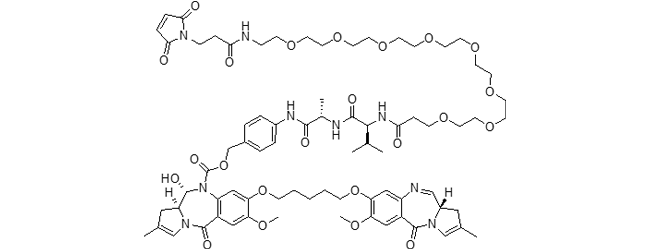
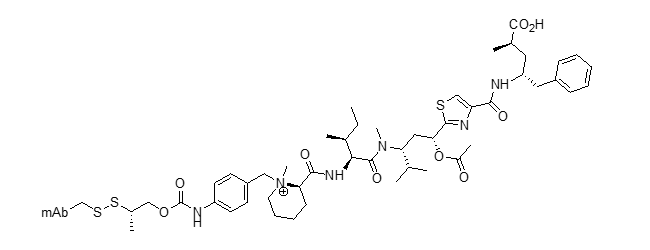
SG3249 ADC (tesirine)
Blood 2018, 131, 1094–1105 DOI: 10.1182/blood-2017-10-813493
Mol. Cancer Ther. 2016, 15, 2709–2721 DOI: 10.1158/1535-7163.MCT-16-0233
Cancer Res. 2018, 78 (13 Suppl.), 2792A DOI: 10.1158/1538-7445.AM2018-2792A
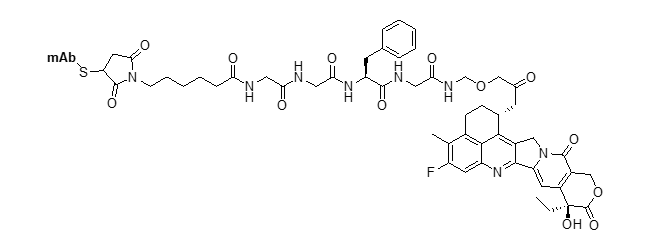
mc-GGFG-DXd ADC (deruxtecan)
Clin. Cancer Res. 2016, 22,5097–5108 DOI: 10.1158/1078-0432.CCR-15-2822
Oncogene 2019, 38, 1398–1409 DOI: 10.1038/s41388-018-0517-4
J. Clin. Oncol. 2018, 36 (15 Suppl.), e24206 DOI: 10.1200/JCO.2018.36.15_suppl.e24206
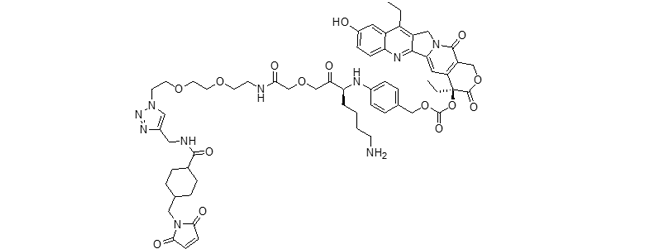
CL2A-SN38 ADC (govitecan)
Bioconjug. Chem. 2015, 26, 919–931 DOI: 10.1021/acs.bioconjchem.5b00223
Engl. J. Med. 2019, 380, 741–751 DOI: 10.1056/NEJMoa1814213
Clin. Genitourin. Cancer 2016, 14, e75–e79 DOI: 10.1016/j.clgc.2015.10.002
Clin. Cancer Res. 2015, 21, 3870–3878 DOI: 10.1158/1078-0432.CCR-14-3321
Mol. Cancer Ther. 2018, 17, 196–203 DOI: 10.1158/1535-7163.MCT-17-0442
Mol. Pharm. 2015, 12, 1836–1847 DOI: 10.1021/mp5006195
J. Clin. Oncol. 2017, 35, 2141–2148 DOI: 10.1200/JCO.2016.70.8297
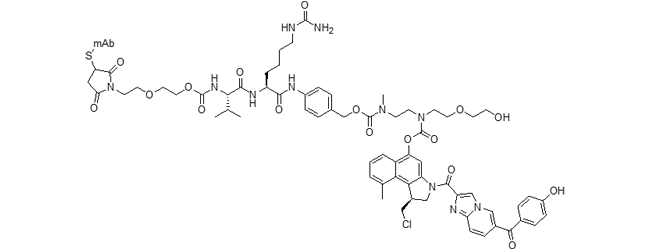
vc-PABC-EDA-seco-DUBA ADC (duocarmazine)
Mol. Pharm. 2015, 12, 1813–1835 DOI: 10.1021/mp500781a
Mol. Cancer Ther. 2015, 14, 692–703 DOI: 10.1158/1535-7163.MCT-14-0881-T
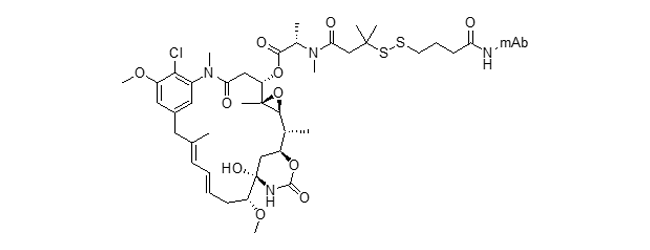
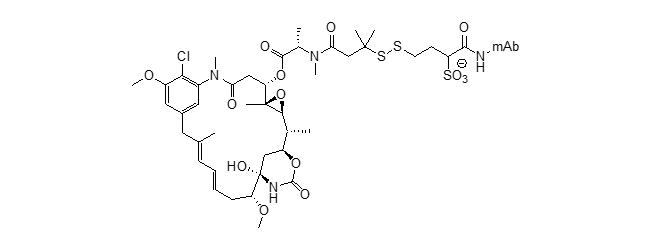
SPDB-DM4 ADC (ravtansine)
Mol. Cancer Ther. 2015, 14 (12 Suppl. 2), C165. DOI: 10.1158/1535-7163.MCT-18-0529
Mol. Cancer Ther. 2014, 13, 1537–1548. DOI: 10.1158/1535-7163.MCT-13-0926
Clin. Cancer Res. 2009, 15, 4028–4037. DOI: 10.1158/1078-0432.CCR-08-2867
J. Clin. Pharmacol. 2017, 57, 865–875. DOI: 10.1002/jcph.869
Cancer Res. 2017, 77 (13 Suppl.), 2630 DOI: 10.1158/1538-7445.AM2017-2630
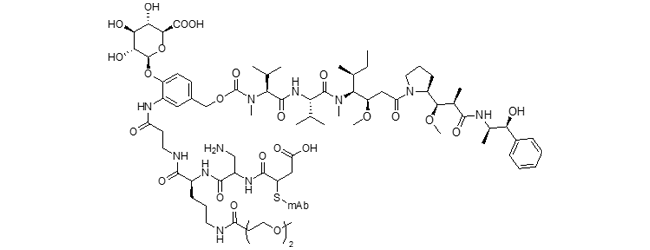
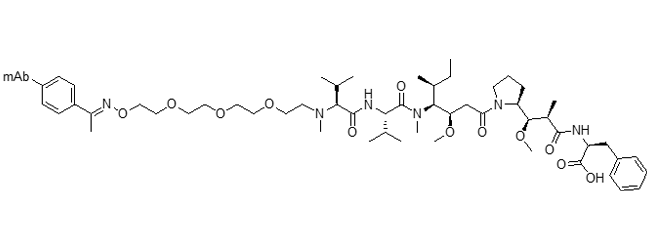
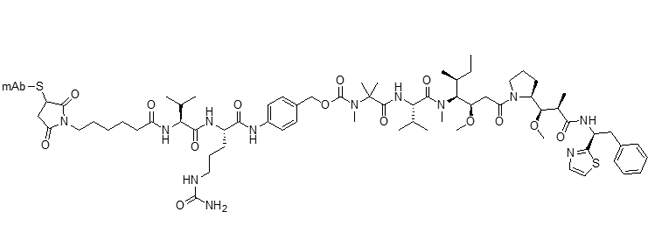
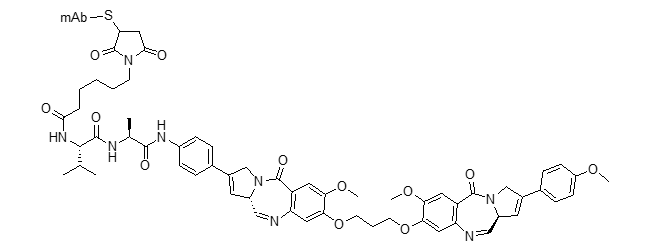
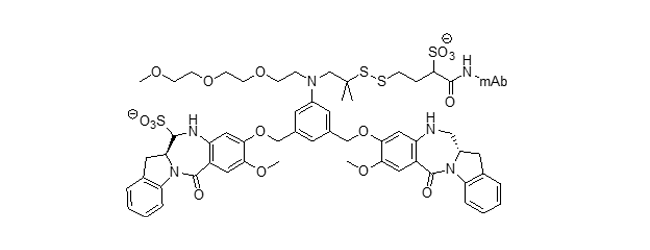
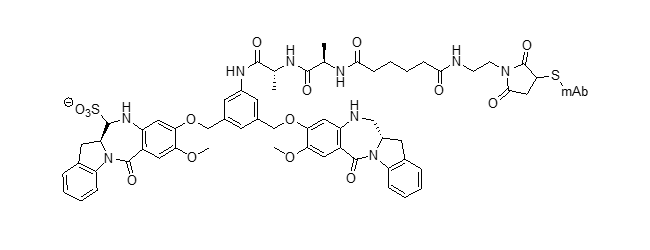
DGN549 ADC
Blood Adv. 2018, 2, 848–858 DOI: 10.1182/bloodadvances.2018017517
Mol. Cancer Ther. 2018, 17 (1 Suppl.), B120 DOI: 10.1158/1535-7163.MCT-19-1102
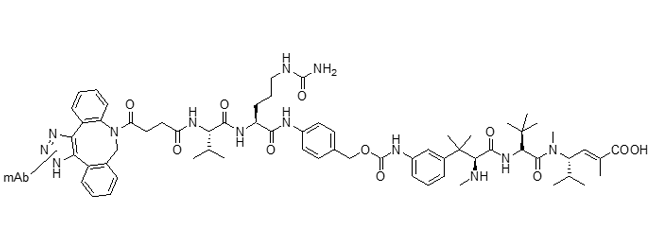
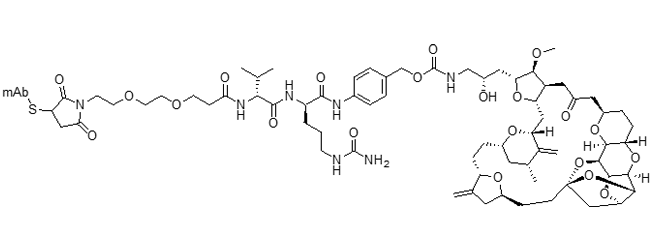
Mal-PEG2-vc-PABC-eribulin ADC
Mol. Cancer Ther. 2018, 17, 2665–2675 DOI: 10.1158/1535-7163.MCT-17-1215