Request a Quote
Bioconjugation Services

Last updated on 28th December 2023
Affordable, Customizable and Expert Solutions
NJ Bio has strong bioconjugation expertise with a broad range of proteins, linker-payloads, and conjugation technologies. We offer different service options to maximize value for our clients and perform bioconjugations at a stage-appropriate level. Our purified conjugates are accompanied by robust analytics to ensure products are well-characterized and have the stated purity. We can help in the selection of suitable linker-payloads that fit the biology of your target.
Discovery Service Options
Express
Conjugation
Standard
Conjugation
Narrow bioconjugation specification
Application: In vivo studies
Custom
Conjugation
Custom linker-payloads
Variety of DAR evaluations
Comprehensive bioconjugation services for all your research needs
NJ Bio’s bioconjugation services refer to specialized solutions offered by our experts in the field of protein chemistry and synthetic chemistry. Bioconjugation services involve the precise and controlled coupling of biomolecules, such as antibodies, proteins, enzymes, or other bioactive compounds, with various chemical entities, nanoparticles, or surfaces. Bioconjugation experts leverage their expertise to design, optimize, and perform custom conjugation reactions, resulting in tailored bioconjugates with enhanced functionality and stability. These services are essential for applications in antibody drug conjugates, diagnostics, drug development, targeted therapies, and research, facilitating the creation of novel tools, assays, and therapeutic agents. By entrusting bioconjugation to our experienced professionals, clients can ensure the successful integration of biological and chemical components, achieving specific objectives in their projects.
Express Conjugation: Early discovery and proof of concept (only for ADCs)
When you need an expeditious and cost-effective determination of whether you have an ADC target, Express Conjugation is an excellent option. The Express Conjugation service allows you to quickly assess your target(s) using approved linker-payloads and stochastic lysine- or cysteine-based conjugation. In general, our turnaround time is 3 to 4 weeks, and we require about 5 to 10 mg of mAb to produce between 2 to 5 mg of well-characterized ADC (with SEC, MS, UV and HPLC). The antibody does not need to be humanized at this stage and we can conjugate to a variety of IgG species and isotypes.
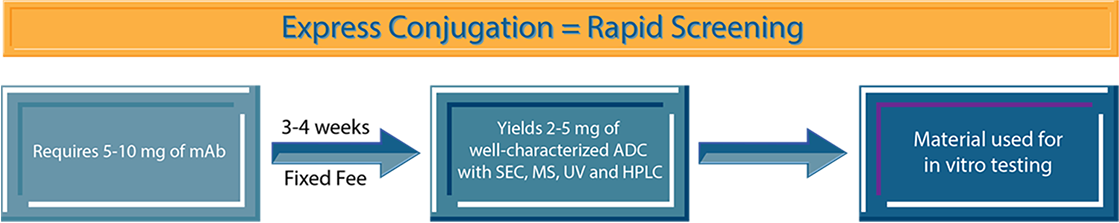
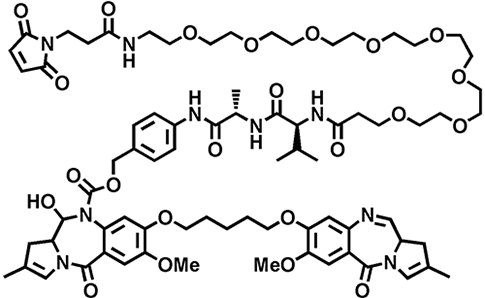
Example: You have 6 murine antibody candidates and want to screen them in vitro with 1 or 2 approved linker-payloads (such as SMCC-DM1, MC-Val-Cit-PAB-MMAE, MC-MMAF, DXd(1) or CL2A-SN38) to help with your selection criteria. NJ Bio can rapidly help you study these leads in vitro and preliminarily in vivo to justify whether to move to the next stage.
Other services offered:
Our team can also perform cell-based functional assays such as cytotoxicity, internalization, and binding assays.
Standard Conjugation
Standard Conjugation is our general conjugation platform for larger scale mAb conjugations and for other protein bioconjugates. Standard Conjugation allows access to a broader range of validated linker-payloads (e.g., DGN549, Tesirine, DM21C, or SPDB-DM4). We offer a fee-for-service option that will deliver larger quantities or conjugations to other proteins. Standard Conjugation is designed to help rapidly validate your target antigen or bioconjugate in vivo. The initial screen may have been carried out with small sampling of linker-payloads and you may need more data. Standard Conjugation will provide material for in vivo selection and narrow the payload release mechanism and mechanism of action (MOA). For a more complete project understanding, measuring internalization rates and antigen numbers will help with selection of linker-payloads. At this stage, we recommend utilizing validated payloads with stochastic conjugation but can also explore site-specific conjugation technologies. The goal of Standard Conjugation is to help you understand the effect of the MOA, the release mechanism, and the potency range required specifically for your biological target.
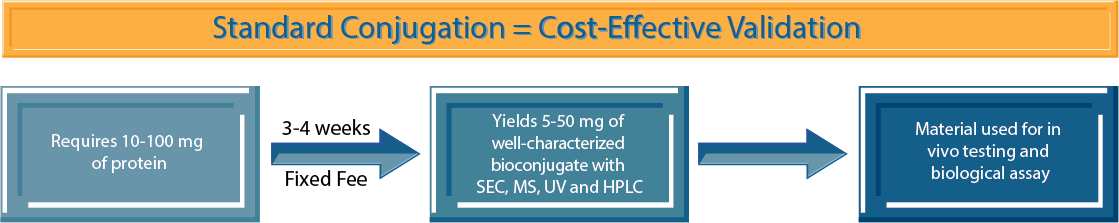
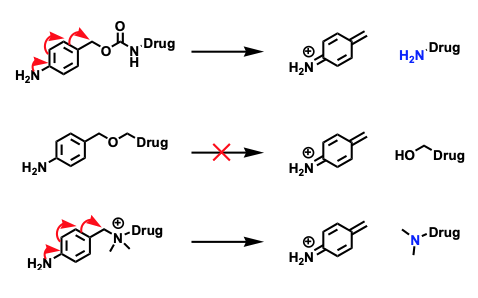
Example: You have proof of concept with MC-VC-MMAE (Vedotin) that shows in vitro activity and some activity in vivo. You would like to assess payloads with different mechanisms of action such as topoisomerases or DNA damaging agent(s) in a larger in vivo study with a dose response. The Standard Conjugation option can help ascertain whether you will have a clinical candidate with an optimized ADC.
Other services offered:
We can conduct in vitro stability of the ADC in a mouse and/or human serum as an add-on and perform cell line validation techniques such as antigen numbers.
Custom Conjugation
You have an ADC and a target that shows clinical promise, but many questions remain to be answered. The Custom Conjugation platform will allow you to determine the best bioconjugate for preclinical development and lead optimization. Validated payloads do not always have the best therapeutic index (TI) for a particular indication. The Custom Conjugation development approach can identify the best conjugate to maximize clinical benefits, based on the biology of your indication. We can mix and match different payloads, linker technologies, and site-specific technologies to obtain the best candidate within your time constraints. We work to maximize stability, increase hydrophilicity, and ensure proper PK and release kinetics. This approach is cost- and time-effective to ensure that the best conjugate is brought forward. During lead optimization, larger quantities of material are needed for toxicology studies, which also requires larger quantities of linker-payloads. Through our flexible FTE program, resources are allocated as needed to efficiently provide high-quality material to keep the project moving forward rapidly.

Example: You have an ADC that shows tumor regression, but the TI is narrow. To maximize clinical success, an improvement to the TI is beneficial. This can be done with testing different site-specific technologies, increasing the hydrophilicity of the linker-payload, and ensuring the ADC is optimized for the expected clinical dose range.
Other services offered:
NJ Bio can perform analyses of animal PK studies, in vitro and in vivo stability, and other cell-based assays to guide lead optimization.
Common Bioconjugation Requests
Protein and Peptide Drug Conjugates
The use of drug conjugates has focused on antibodies, but other proteins and peptides can also be used for targeted delivery depending on the indication and the role. Proteins and peptides have a great diversity of structures, and conjugation technology developed for antibodies may not transfer to all types of proteins. For example, the hinge disulfides of antibodies are commonly used as a conjugation group, but many proteins do not have accessible disulfides or available cysteines. This would mean that cysteine conjugation is not always possible with all proteins. However, in most cases, lysine conjugation is available for initial screening. NJ Bio can adapt ADC technology to non-antibody proteins.
An advantage of using smaller proteins and peptides is that these smaller-sized conjugates can penetrate the tumor more easily and are less prone to antigen barrier effects. Smaller proteins can be easier to produce than antibodies, especially if they are made in bacterial expression systems. Peptide conjugates are comparatively smaller and can have a better profile in terms of cellular uptake and tissue penetration. Common motifs used in protein and peptide drug conjugates are bicyclic peptides, knottin, affibody, adnectin/centyrin, DARpins and antibody fragments. These conjugates are being investigated as both delivery vehicles and as imaging agents. The difference between conjugating smaller peptides and proteins compared to antibodies is that the drug-linker has a larger impact on overall properties. Antibodies can have drug to antibody ratio (DAR) of up to 8; such a high DAR is unlikely with smaller peptides without causing aggregation. Another aspect of conjugating to smaller proteins and peptides is that the drug-linker can also more easily interfere with binding. With smaller proteins and peptide conjugates, the linker position, the properties of the drug-linker, and the DAR are three elements that need to be carefully balanced.
NJ Bio has experience with the conjugation and purification of smaller proteins and peptides and has helped many clients validate their smaller targeting protein platforms. Our analytical techniques and equipment are adapted for smaller conjugates. We have substantially invested in mass spectrometry expertise and equipment, which apply well to smaller conjugates. We design these molecules by employing our thorough expertise in conjugation chemistry and deliver high-quality bioconjugates with improved properties.
Carrier Protein Conjugates
NJ Bio has experience in the attachment of proteins to carrier agents that elicit an immune response. Common carrier agents include BSA (bovine serum albumin), KLH (keyhole limpet hemocyanin) and OVA (ovalbumin). These carriers are often designed to generate an antibody response to a linked antigen and are important tools in antibody discovery. We have experience in the conjugation of proteins to carrier agents, with applications from vaccines to antibody generation.
Oligonucleotide Conjugates
NJ Bio has specialized expertise in ADCs as well in nucleotide chemistry. This experience in both fields is an important asset in the emerging field of oligonucleotide conjugation. Oligonucleotides precisely target the DNA/RNA sequence they are designed to inhibit, but targeted delivery to cells is required due to their lack of membrane permeability, short PK, and lack of stability. Conjugating oligonucleotides to antibodies and other targeting motifs has been a rapidly growing field in the past 10 years. Oligonucleotide conjugates are not only potential therapeutics but also great diagnostic agents that have shown high sensitivity and excellent selectivity. Oligonucleotide conjugates have different properties and challenges compared to smaller molecules; thus, the techniques are not always transferable. NJ Bio can deliver oligonucleotide conjugates for a variety of applications and constructs.
An example of a difference between ADCs and oligonucleotide conjugates is linker compatibility. Not all linkers compatible with small molecules are compatible with oligonucleotides. The position, linker type and even the sequence of adding the linker before or after the annealing step can impact the conjugation efficiency and yield. Our experience in nucleotide chemistry allows us to understand the reactivity and compatibility between different groups at the molecular level.
We have also found that an important asset in working with oligonucleotides is the availability of robust analytical instruments and methods. The quality of the conjugates is only as good as the analytics that accurately represent the products. Sometimes the conjugate is made successfully, but if analyzed with the wrong method, the experiment can appear unsuccessful. This can lead to the need for further troubleshooting and added experiments, for instance when the conjugate is filtered on the column, it does not ionize well in the MS method, or the staining is weak. The charge and size of oligonucleotides leads to a different purification workflow compared to more traditional small molecule conjugation. Oligonucleotides are big and cannot always be easily removed from the conjugate by size-exclusion methods. Both ion-exchange and size-exclusion are often necessary to obtain high quality material.
NJ Bio has invested in mass spectrometric methods which have been very valuable for oligonucleotide conjugate platforms, especially when it comes to troubleshooting. Instruments and methods for oligonucleotides are now well-established at NJ Bio, given our extensive experience in this space. The field of oligonucleotide conjugates is growing and will require innovative solutions. NJ Bio is equipped to provide these solutions.
NJ Bio has published a review article entitled Antibody-Oligonucleotide Conjugates: A Twist to Antibody-Drug Conjugations. A summary of the key technological advancements in the preparation of antibody-oligonucleotide conjugates (AOCs) and the distinct advantages and disadvantages of AOCs as novel therapeutics are presented.
Examples of Antibody Oligonucleotide Conjugates
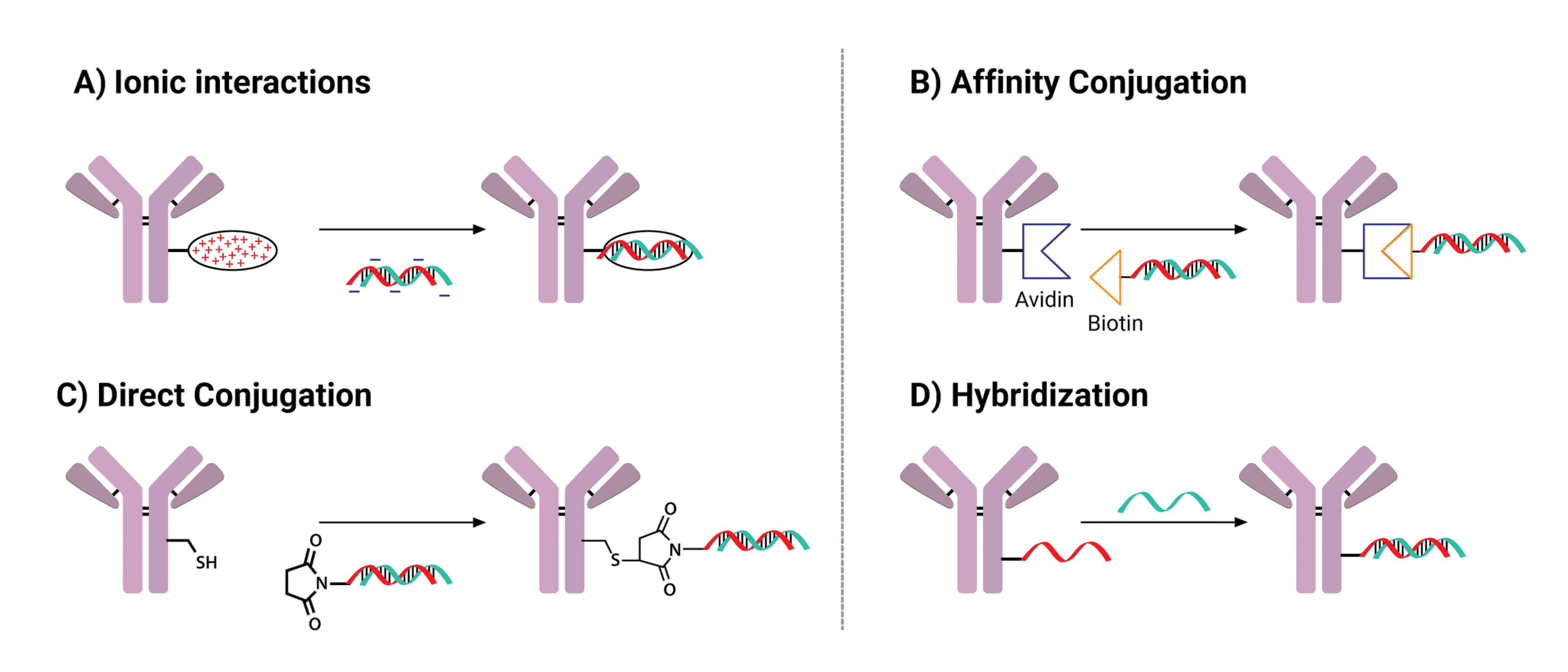
Dugal-Tessier, J.; Thirumalairajan, S.; Jain, N. Antibody-Oligonucleotide Conjugates: A Twist to Antibody-Drug Conjugates. J. Clin. Med 2021,10, 838.
Other Conjugates (e.g., fluorescent dye-labelled, biotinylated, derivatized with chelators)
At NJ Bio, we have experience in the labelling of proteins for other applications. Conjugation of dyes that allow tracking by fluorescence or by microscopy is routinely performed. Biotinylation is an important tool for the capture of specific proteins for analysis by ELISA and mass-spectrometry. Our chemistry experience is an asset when a linker-dye combination is not commercially available, allowing us to perform the synthesis and conjugation under one roof.
Development Services
Process
Development
DOE and scale-up to support preclinical development
Prequalification studies
PK/PD assay development
Clinical Material
Manufacturing
Phase I and Phase II material
(Upcoming soon. Please contact us for details.)
cGMP suite
Technical transfer
(Upcoming soon. Please contact us for details.)
Process Development (Bioconjugation and Linker-Payload Synthesis)
If you are looking for Process Development support, our scientists have worked on projects that span the research and development spectrum. We have experience supporting the development of ADCs by optimizing, performing Design of Experiments (DOE), and scaling up material needed for preclinical development. We can scale up the chemistry of the linker-payload in-house, which will allow technical transfer as needed to a partner of your choice. The process we develop for your conjugation and linker-payload chemistry is assured to be robust for such transfers.
Our bioanalytical team can develop and validate methods during the preclinical stage that can be adapted and transferred to a clinical testing lab. The advantage of working with NJ Bio is all preclinical development activities can be performed under one roof.
Clinical Material Manufacturing
A cGMP suite in Princeton, NJ is scheduled to be online in 2Q 2024 to support the production of Phase 1 and Phase 2 clinical trial materials. Our smaller-footprint facility will allow production of early clinical trial materials more efficiently and cost-effectively than larger commercial suppliers. Our goal is to have a seamless transition across method development, engineering runs, and production of key clinical materials.

Anatomy of an Antibody Drug Conjugate (read more)
Click here for review articles on Antibody Drug Conjugates