Request a Quote
Manufacturing Services

Last updated on 29th February 2024
Comprehensive, Scalable and Advanced Manufacturing Solutions
NJ Bio offers state-of-the-art contract development and manufacturing (CDMO) services specializing in Antibody-Drug Conjugates (ADCs), novel payload-linkers, and mRNA for preclinical and clinical studies. We offer a comprehensive suite of services for development and clinical manufacturing, designed to accelerate our clients’ research programs. Our team of scientists and experts is committed to providing services tailored to the specific requirements of each project. From synthesis to purification, we maintain precision and efficiency at every stage. NJ Bio possesses the expertise to optimize processes in terms of yield and cost of production of clinical-grade materials. Our flexible and scalable drug development solutions were designed with ADCs, bioconjugation and mRNA in mind to support diverse research projects and enable our clients to efficiently transition from clinical candidates to clinical assets at an accelerated pace.
GMP Manufacturing Capability
Clinical manufacturing or clinical trial material manufacturing (CTM) is the process of manufacturing therapeutics to support clinical research. NJ Bio’s GMP suites are equipped and specifically designed to manufacture Phase 1 and Phase 2 clinical trial materials, which offer a more cost-effective solution compared to larger commercial suppliers. The strategic positioning of our R&D and manufacturing facilities enables a smooth transition throughout the entire process — from screening molecules, method development and engineering runs to the production of clinical materials. This integrated project management approach ensures consistent delivery of high-quality products that meet the most stringent quality standards.
Our facility has been purposefully constructed to meet the standards of international regulatory boards and to efficiently scale up production to fulfill the increasing requirements of clinical trials. Single-use technology is employed for various processes, including solution preparation, synthesis, downstream chromatography, and filtration. Single-use or disposable equipment, especially in the production of ADCs, reduces the likelihood of cross-contamination, while simultaneously enhancing product efficiency by shortening product changeover durations, intra-batch cleaning, and maintenance cycles. 1
NJ Bio has implemented a comprehensive quality management system in strict adherence to current Good Manufacturing Practice (cGMP) regulations. The facility encompasses both pilot and clinical scale capabilities, and we have implemented a Phase-appropriate Compliance (PAC) quality system to meet the regulatory standards set forth by agencies such as FDA, EMA, MHRA and others. This system not only ensures compliance but also enhances the efficiency, effectiveness, and credibility of our processes.
Antibody-Drug Conjugate Manufacturing Services
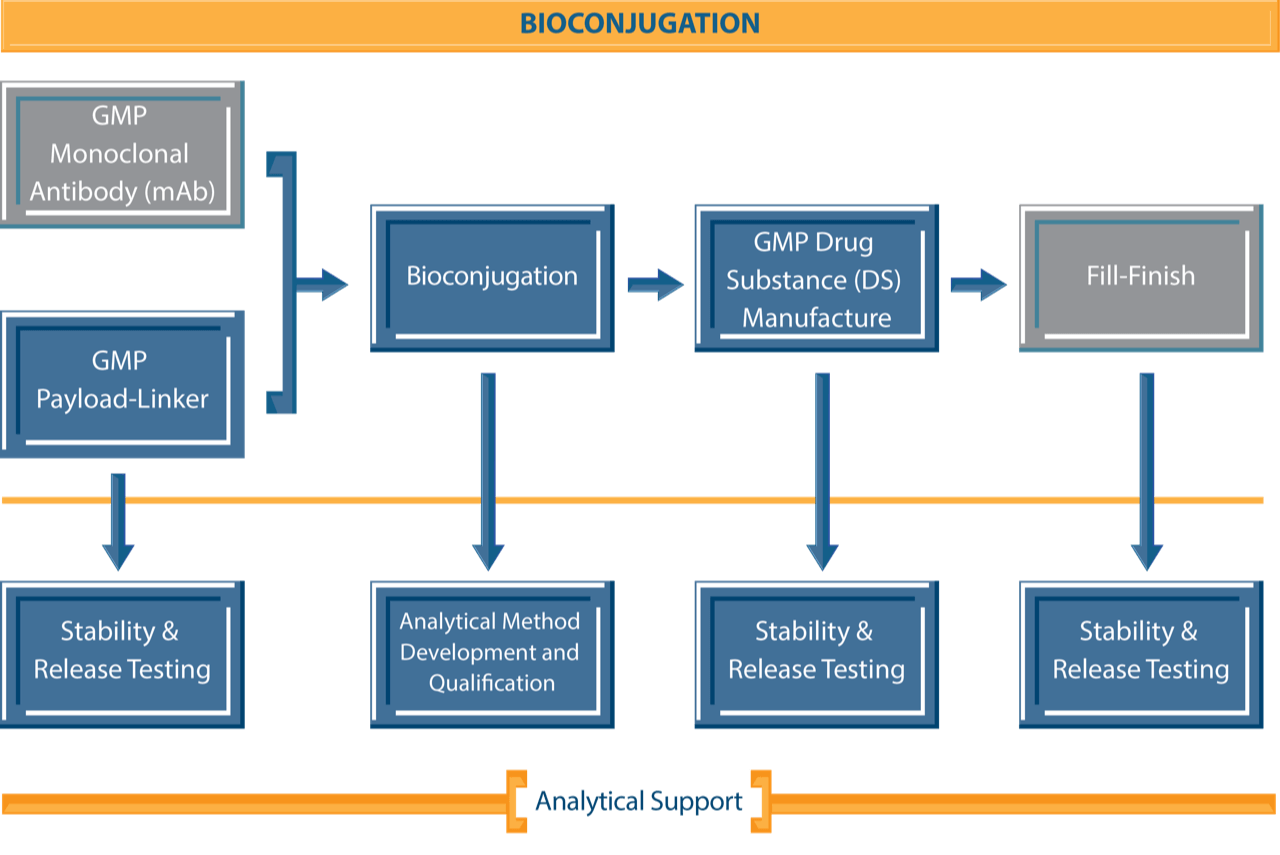
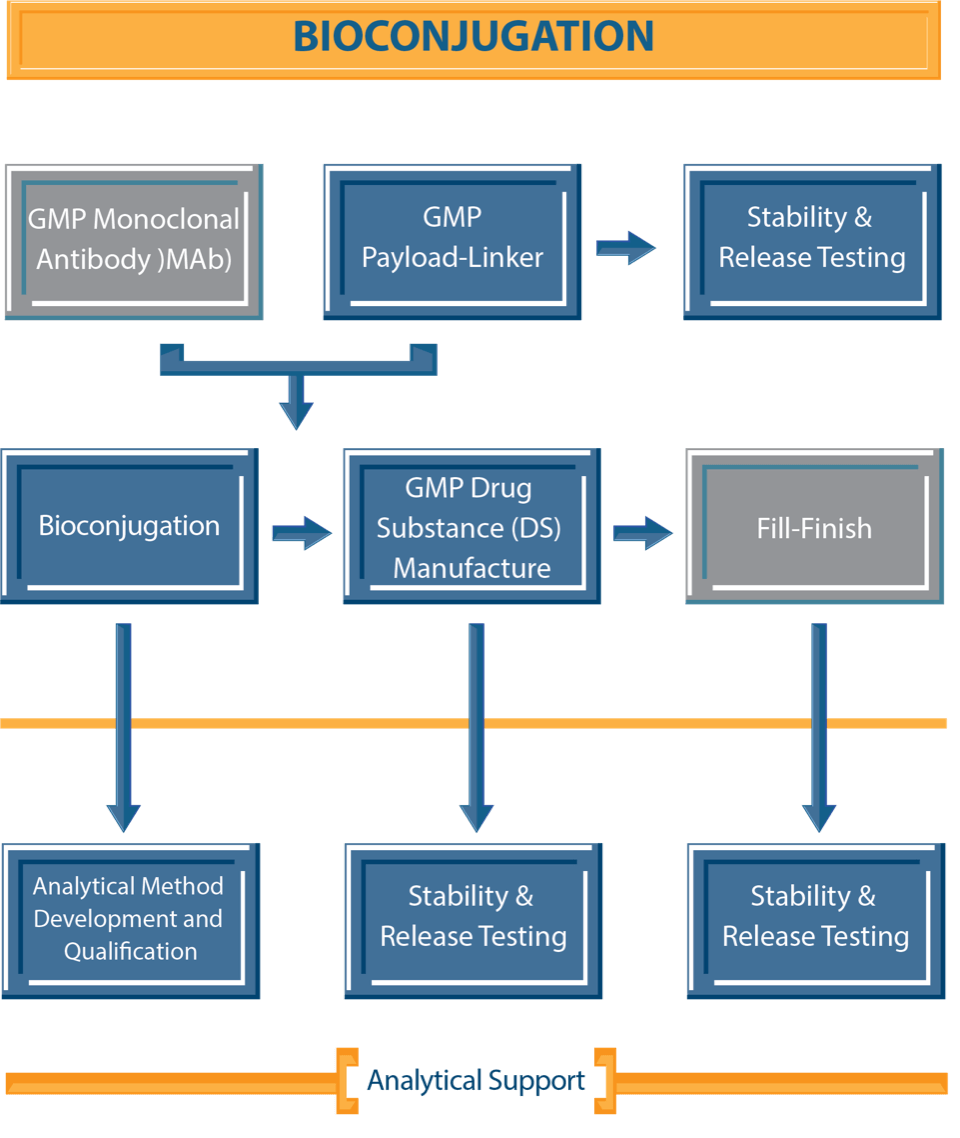
NJ Bio’s ADC development and manufacturing facility is designed to accelerate the entire process from ADC selection to production of clinical grade materials. Our facility is well-equipped to handle the intricate processes integral to ADC production. With a strong expertise in bioconjugation and synthetic chemistry, our team can address challenging issues encountered during the development and manufacturing of investigational ADCs for clinical trials thus expediting the entire drug development process. A state-of-the-art facility, strong experience in early research and development, and extensive knowledge of novel linkers, payloads, and conjugation technologies, enables us to streamline the drug development pipeline, ensuring a smooth transition from discovery to clinical material production of novel therapeutics.
Our high potency lab is designed to handle derivatives or analogs of highly potent cytotoxic agents, whether of natural or of synthetic origin, such as auristatin, maytansinoids, duocarmycins, and more. We provide extensive services for ADC development including but not limited to process development and optimization, bioconjugation, novel linker-payload technology and production of ADC drug substance (DS).
We also develop robust analytical methods to assess the purity, stability, and quality of produced ADCs, offering an advanced bioanalytical testing platform. Stringent quality assessment measures are implemented for in-process materials, ensuring consistency of the ADC product.
mRNA Manufacturing
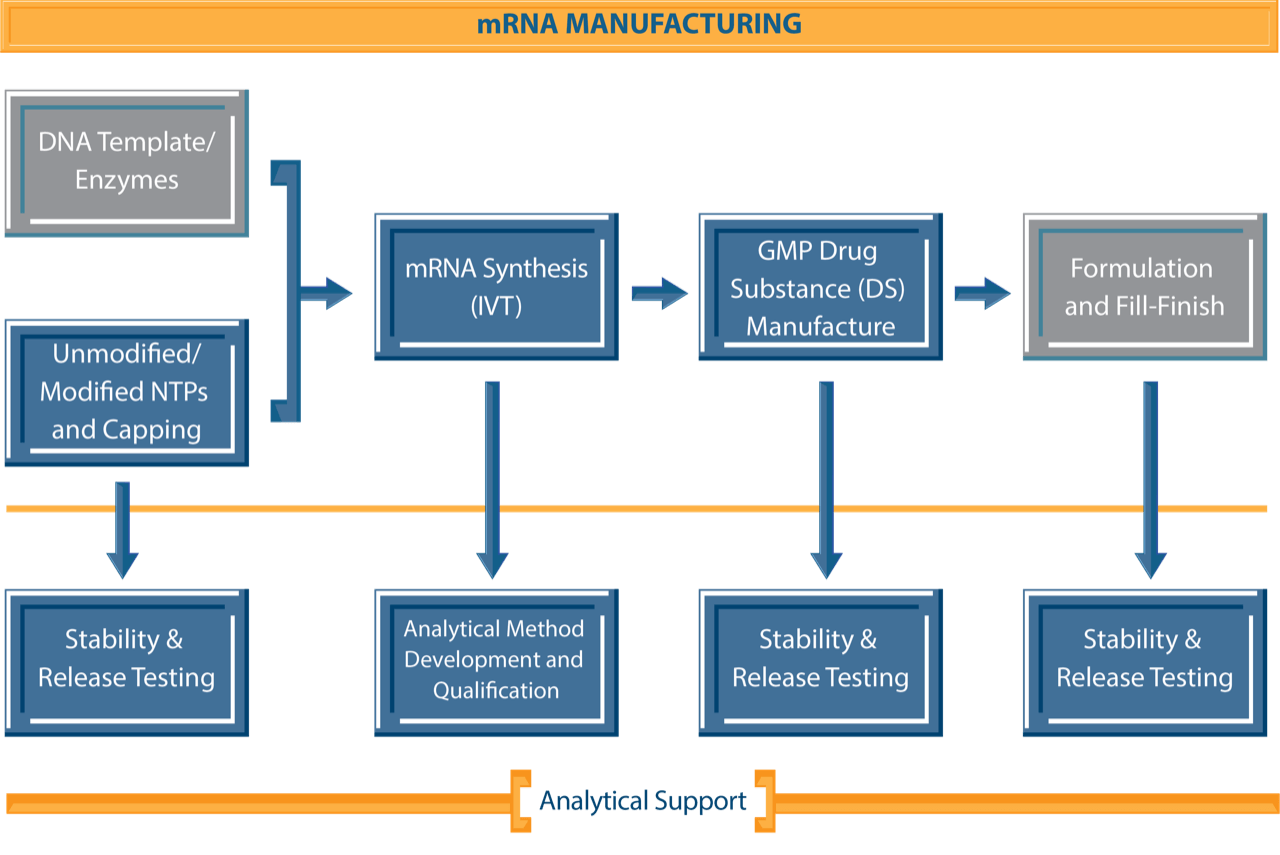
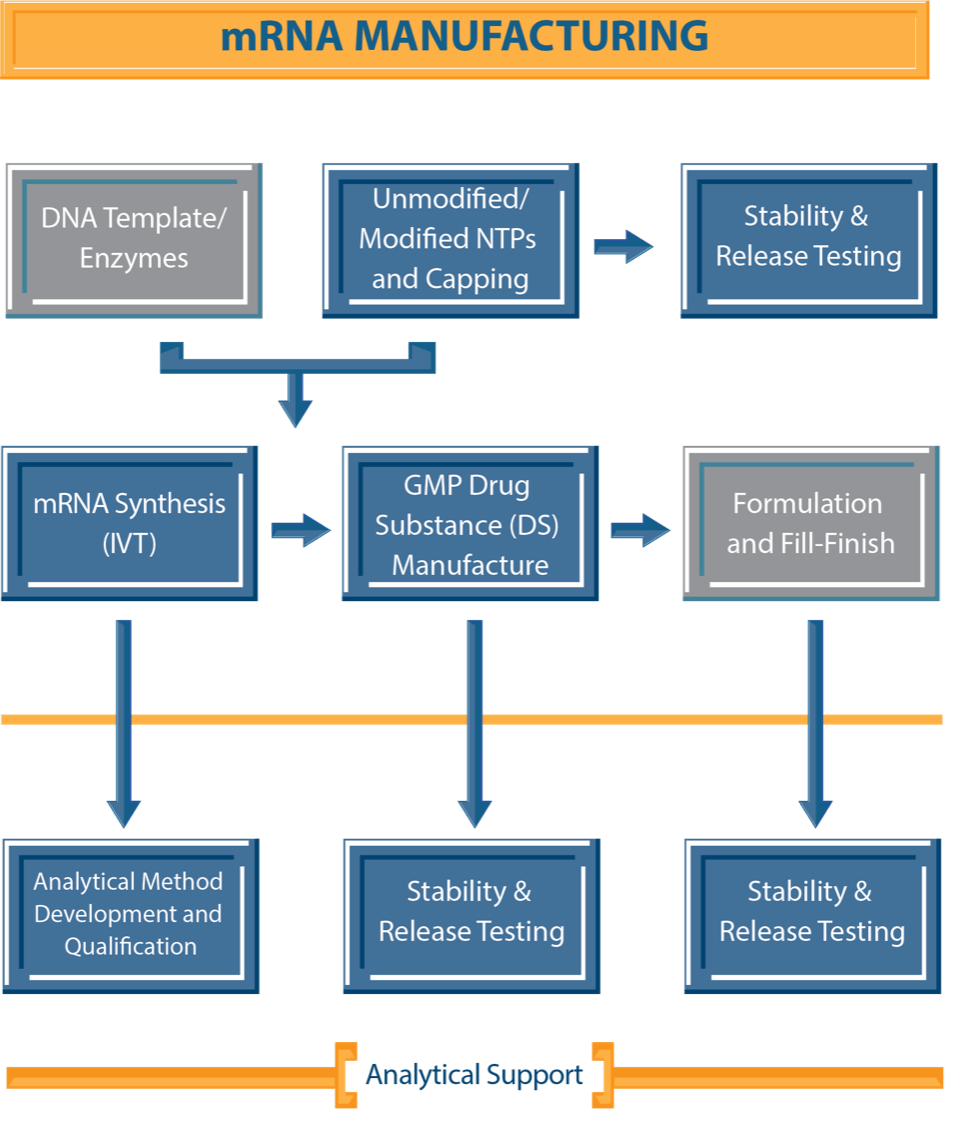
NJ Bio’s combined expertise in chemistry and biology enables us to deliver quality solutions for the development and manufacturing of custom mRNA sequences. Our team specializes in customizing functional mRNAs, carrying out mRNA synthesis via in vitro transcription (IVT), producing unmodified/ modified NTPs and capping agents, and provides comprehensive technical support throughout the research program. Leveraging our expertise, we can produce high-fidelity mRNA sequences of diverse lengths and functions, ensure optimal expression, mitigate immunogenicity concerns, and enhance the overall stability of the mRNA product to align specifically with our clients’ project requirements.
Payload – Linker Manufacturing
NJ Bio provides an integrated suite of services encompassing discovery, development, and GMP manufacturing specifically for chemical payloads and linkers. Our capabilities include performing a wide range of chemical reactions, as well as handling isolation, purification, and analytical method development and validation for release and stability testing. At our GMP high potency lab we can produce chemical payloads in gram- to kg-scale,
We are adept in developing and scaling up processes related to small molecules and payloads designed for ADCs, custom synthesis of novel linker-payload variants, strategic designing of linker-payloads, and executing process development for the seamless transition from development to scale-up and manufacturing. We consistently work towards simplifying synthetic routes, identifying optimal linkers, and synthesizing novel payloads with high potency.
Developing and scaling up payloads pose significant challenges due to their cytotoxic nature and complex molecular structure. The intricate synthetic routes required for their production are time-consuming and expensive. Moreover, the necessity for high-containment facilities adds complexity to both development and manufacturing. Traditional synthetic methodologies used by chemists are often inadequate for these toxic payloads. Consequently, expertise in optimizing synthetic routes becomes crucial to enhance cost efficiency, quality, and manufacturing timelines.2 Our team specializes in the development and optimization of synthetic routes, particularly for the highly toxic molecules used in ADCs.
Our manufacturing services include;
- Process Development: Developing and refining processes, alongside creating analytical methods to ensure quality and consistency.
- GMP Manufacturing: Providing services at varying scales to support clinical studies.
- Analytical Method Development and Qualification: Establishing and qualifying analytical methods for release tests and stability studies to guarantee product quality.
- High-Potency Handling: Advanced facility equipped for manufacturing and testing highly potent compounds using various techniques such as IR, HPLC, GC, and more.
References
(2) Goundry, W. R., & Parker, J. S. (2022). Payloads for antibody–drug conjugates. Organic Process Research & Development, 26(8), 2121-2123. https://doi.org/10.1021/acs.oprd.2c00227
Frequently Asked Questions (FAQs)
The timeline varies based on project scope. On average, the GMP manufacturing process for ADCs can take approximately 12 months from initiation to GMP release.
Does NJ Bio offer stability testing for the manufactured products?
NJ Bio offers GMP release & GMP stability testing ensuring quality, safety, and efficacy of the product. Our stability testing includes long-term stability studies (25°C ± 2°C/60% RH ± 5% RH, 2- 4 °C, – 20 °C & -80 °C), intermediate stability studies (30°C ± 2°C/65% RH ± 5% RH) and accelerated studies (40°C ± 2°C/75% RH ± 5% RH) as per ICH guidelines. We provide a detailed stability report to our clients and also offer GMP reference standard qualification services.
Does NJ Bio offer analytical development services for the products?
NJ Bio has a dedicated and experienced analytical team focused on developing robust analytical methods to characterize small molecules (payload, payload-linkers, modified NTPs, etc.), mAbs/ADCs (protein, peptides, mAbs, oligopeptide/protein conjugates) and mRNA for the critical quality attributes. Our analytical development team utilizes a range of techniques, including HPLC/UPLC instruments with UV/PDA/ELSD/RI/MALS/SQD detectors, high resolution mass spectrometry instruments (multiple Waters Xevo G3 QTOF, Xevo G2 QTOF & TOF), highly sensitive mass spectrometry instruments (TQ-S, TQ-D, TQ-absolute, Sciex Triple Quad) PA 800 Plus, ProteinSimple Maurice, NanoDSC and other spectroscopic instruments such as soloVPE, UV-VIS, Karl-Fisher & FTIR.
Do your manufacturing processes adhere to the regulatory requirements of key agencies such as the FDA, EMA, MHRA, and other relevant authorities?
Yes, our manufacturing processes are designed and would be executed with strict adherence to regulatory standards, including those set by the U.S. Food and Drug Administration (FDA), the European Medicines Agency (EMA), and the Medicines and Healthcare Products Regulatory Agency (MHRA).
Do you use single-use equipment in your GMP manufacturing processes?
Yes, our manufacturing processes primarily rely on single-use equipment to ensure product quality and streamline operations.



